Abstract
Introduction
Despite the accepted utility of autologous stem cell transplantation for multiple myeloma, the role of allogeneic stem cell transplantation (alloSCT) remains controversial (Dhakal et al., 2016). The differences in practices across centres in patient selection, conditioning regimens and post-transplant care clouds the comparison of available studies. Moreover, the high rates of non-relapse mortality despite disease eradication demand an understanding of pre-allograft determinants of long-term survival. Included within these determinants is the appropriate timing for alloSCT, an important variable given the growing list of available myeloma therapies. We sought to identify the variables, including the influence of transplant centre and timing that govern long term survival following alloSCT for myeloma within integrated data in a large group of patients across three centres.
Methods
A retrospective analysis of 93 patients undergoing alloSCT for multiple myeloma between January 2000 and June 2017 at three tertiary transplant centres - the Royal Melbourne Hospital, Australia and The Christie and Central Manchester NHS Foundation Trusts, United Kingdom. We collected: demographic data; disease-specific data, including previous treatment, disease status prior to alloSCT, and time from autologous SCT to alloSCT; and peri-transplant data including donor source and gender, stem cell dose, conditioning regime, and donor and recipient cytomegalovirus serological status. We measured overall survival, cause of death, acute and chronic graft versus host disease and progression free survival. Log-rank testing and cox regression analysis was performed with a multivariate regression model established on the basis of significant univariate associations.
Results
Median age for the 93 patients at transplant was 49 years old (range 28 to 66 years). 65% of patients were male (n=60). 51 patients underwent alloSCT at the Royal Melbourne Hospital, 18 and 24 patients at the Christie and Central Manchester NHS Foundation Trusts, respectively. Patients received a median number of 2 lines of treatments prior to alloSCT (range 0-6). 51% (n=47) patients received stem cells from voluntary unrelated donors (VUD), and 49% from siblings (n=46). 78% (n=72) received a reduced intensity conditioning consisting of either 25mg/m2 fludarabine, 60mg/kg cyclophosphamide or 30mg/m2 fludarabine with 2Gy total body irradiation; 20% (n=18) received non-myeloablative conditioning with 30mg/m2 fludarabine and 750mg/m2 cyclophosphamide, 2% (n=3) underwent myeloablative conditioning with 60mg/kg cyclophosphamide and 4Gy total body irradiation.
Median overall survival (OS) for the whole group was 8.1 years (interquartile range = 1.3 to 12.7 years). No difference was observed in OS across the three centres (log rank p=0.84) or across country (log-rank p=0.56). Conditioning regimens containing total body irradiation showed no association with relapse post allo SCT (odds ratio 0.68, 95% CI 0.30-1.55) and did not affect OS (hazard ratio, HR =1.00, 95% CI = 0.51-2.00).
In accordance with previous findings (Dhakal et al., 2016), age over 40 at alloSCT was associated with a poorer survival (HR = 2.47, 95%CI 1.20 to 5.09). Patients who had stable or progressive disease at allograft showed poorer OS, even when controlling for number of lines of therapy and previous relapse (HR = 3.03, 95% CI 1.31-6.97).
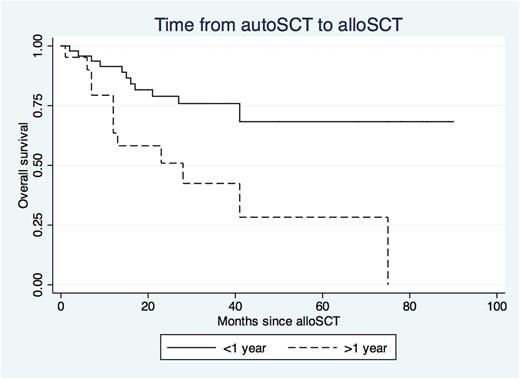
Patients who underwent alloSCT over one year following autologous SCT showed 3.4 times increased risk of death (95% CI 1.52-7.09) and 2.16 times increased risk of relapse (95% CI 1.18-3.93) even when controlling for age, prior relapse history, number of lines of treatment, disease status prior to alloSCT and transplanting hospital (Figure 1).
Conclusion
This large multi-centre multivariate analysis demonstrates that transplant centre and low dose total body irradiation does not influence OS following alloSCT for multiple myeloma. Importantly, the time between autologous SCT and alloSCT of greater than one year is a strong predictor of poor OS. This remains true when controlling for several established risk factors. Our results demonstrate the importance of patient selection and timing in order to maximise OS for patients undergoing alloSCT for multiple myeloma.
Reference
Dhakal B, et al. 2016. Allogeneic stem cell transplantation for multiple myeloma: is there a future? Bone Marrow Transplant, 51, 492-500.
Routledge: Gilead: Honoraria; Celgene: Honoraria; Bristol-Myers Squibb: Honoraria. Harrison: Celgene: Consultancy, Research Funding, Speakers Bureau. Cavet: Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Speaker, Research Funding. Tholouli: Pfizer: Honoraria, Speakers Bureau; MSD: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Honoraria, Speakers Bureau. Ritchie: Amgen Inc.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.